 |
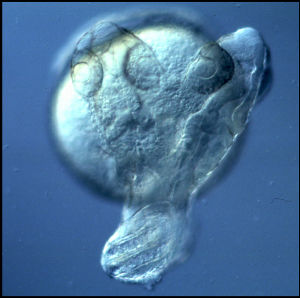
Specification of Embryonic Axes:Embryonic axis formation in vertebrates is initiated by the establishment of the dorsal Nieuwkoop blastula organizer, marked by the nuclear accumulation of maternal ?-catenin, a transcriptional effector of canonical Wnt signaling. At the onset of zygotic transcription, in frog and zebrafish, maternal ?-catenin activates expression of genes that establish the Spemann-Mangold (SMO) gastrula organizer that patterns the germ layers and coordinates gastrulation movements. We demonstrated that the zebrafish homeobox bozozok locus acts downstream of ?-catenin, at the top of a hierarchy of zygotic genes specifying SMO formation and development of the dorso-anterior embryonic structures. G protein-coupled receptors (GPCRs) have also been hypothesized to inhibit ?-catenin and axis formation, however the identity of such GPCRs has remained unclear. Our work showed that zebrafish Ccr7 chemokine GPCR and one of its ligands, Ccl19.1, increase ?-catenin degradation in a Gsk3?-independent manner. In this new model of axis specification, Ccr7 acts as a long-hypothesized GPCR that provides a global inhibition of ?-catenin to ensure the precise formation of embryonic axes (PMC3467228). In our current studies we are defining the underlying mechanisms of Ccr7 action by studying mutants in the Ccr7 and other components of this pathway. |
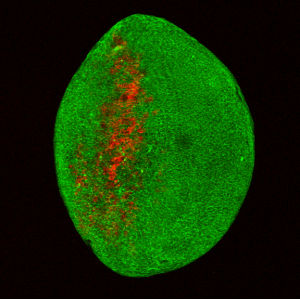 |
Convergence & Extension Movements:During vertebrate gastrulation convergence and extension are key processes that narrow embryonic tissues along the mediolateral embryonic axis while extending them from head to tail. Studies from our and other groups have shown that these movements are driven by several cell behaviors, including directed migration and intercalation of mediolaterally elongated cells. Furthermore, we implicated several pathways in regulation of convergence and extension movements of entire germ layers (non-canonical Wnt signaling, Bone Morphogenetic Proteins, Prostaglandins, G-protein Coupled Receptors). Current projects delineate the cellular and molecular mechanisms via which these pathways determine the specific gastrulation cell movement behaviors. |
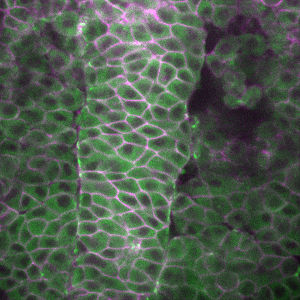 |
Coordination of Embryonic Patterning and Morphogenesis:For completion of normal embryonic development the inductive processes specifying cell fates and gastrulation cell movements that sculpt the body plan must be exquisitely coordinated. Our studies aim at dissecting the coordination of the inductive and morphogenetic processes that establish the vertebrate body plan. Our studies led to a model whereby Wnt/PCP signaling works during gastrulation as a cellular compass that coordinates AP/ML polarity and behaviors of individual cells with embryonic polarity (http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3166557/). WE are starting to define chromatin factors that regulate both cell fate specification and cell movements during gastrulation. |
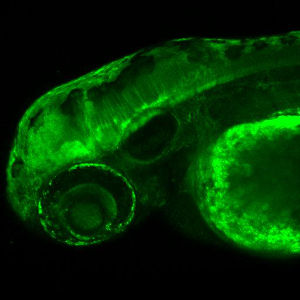 |
Dachsous/Fat Cadherins:Dachsous and Fat atypical cadherins are evolutionarily conserved regulators of planar cell polarity, tissue size and cell adhesion that have been implicated in human diseases. Our recent work in zebrafish uncovered multiple roles of Dachsous in embryogenesis, including the earliest developmental stage, egg activation, including cytoplasmic segregation, cleavages and maternal mRNA translocation, in transcriptionally quiescent embryos (Li-Villarreal et al., 2015; http://www.ncbi.nlm.nih.gov/pubmed/26160902). Our current studies test the hypothesis that Dachsous cadherins affect some developmental processes through regulation of microtubule and actin cytoskeleton, acting independent of its known ligand Fat. |
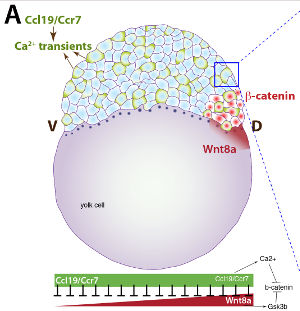 |
G-protein Coupled Receptors:We have recently provided the first evidence that G-protein coupled receptors are required for gastrulation movements of discrete cell populations. Zebrafish homologs of the Agtrl1b receptor and its ligand, Apelin, previously implicated in physiology and angiogenesis, control movements of heart precursors and heart field formation. Moreover, we implicated Ccl19/Ccr7 chemokine GPCR signaling in zebrafish axis formation as a negative regulator of the Wnt/?-catenin pathway. We are now working towards identification of additional G-protein coupled receptors that regulate gastrulation movements, and testing whether the functions of GPCRs we uncover in zebrafish are relevant to human development. |
 |
Forward Genetics:One of the main strengths of the zebrafish model is that genetic screens can be used to identify, in an unbiased way, genes essential for developmental processes. Our lab is engaged in screens for mutations that impair gastrulation, early patterning and lead to scoliosis-like phenotype in adults. |
 |
Reverse Genetics and Genome Engineering:To identify mutations in known genes of interest, we employ a combination of the TALEN and CRISPR/Cas9 nuclease approaches. We have improved methods for homologous-recombination-based gene replacement and knock-ins using TALEN-nucleases and are employing these methods to introduce fluorescent tags and other modifications into endogenous zebrafish genes (Shin et al., 2014; PMC4197590). |
 |
Dissecting Scoliosis in Zebrafish:Adolescent idiopathic scoliosis (AIS) or late-onset scoliosis affects near 3% of the pediatric population, presenting with body curvature without overt structural defects of vertebral units. Despite presenting significant burden to the society, there is limited understanding of the genetic basis of AIS. Having a well-annotated genome and plentiful, transparent progeny, zebrafish afford a powerful vertebrate model to study AIS by employing forward and reverse genetic approaches. We have carried out a pilot genetic screen for chemically induced mutations and uncovered 31 recessive adult mutants with scoliotic features. Ongoing complementation and mapping experiments of 17 recessive scoliosis mutants have defined at least 10 distinct loci. We are employing massively parallel sequencing to identify the affected mutant genes. Towards validating candidate loci identified in human AIS patients and genomic studies, we are leveraging the gene targeting and editing methods we recently helped to improve. |
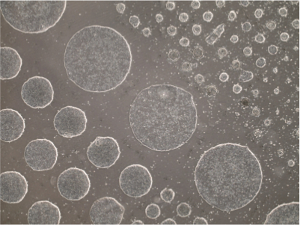 |
Modeling Aspects of Human Development:Pluripotent stem cells afford studying aspects of human development by their directed differentiation into specific cell types, such as cardiomyocytes and neurons, or by generation of embryoid bodies and organoids. We are employing these approaches to test specific hypotheses about gene function or cell behaviors deduced from our studies in zebrafish and gain insights about human development. |
